From this point of view, the theory that transportable Cfragments can serve as additional nutrients is a novel view of acetone metabolism which introduces a new approach to the study of acetone degradation, especially in understanding its physiological function and the interrelationship between liver and peripheral . Vapors can flow along surfaces to distant ignition sources and flash back. Static discharge may also ignite acetone vapors, though acetone has a very high. Сохраненная копия Перевести эту страницу Product Identification, Back to Contents.
Synonyms】 2-Propanone Dimethyl ketone. Boiling Point , 56ºC, Dipole Moment, 2.

Melting Point , – 94ºC, UV cutoff, 3nm. C, Vapor Density, (vs. air). Instrument, IFS66V (Bruker). Dielectric constant, 20. C and boiling point of 56. Acetone has a melting point of −95.
It has a relative density of 0. It acts as a solvent and is readily miscible with other solvents, including water, ethanol, and diethyl ether.
No new substance substance were formed. Why does it evaporate at room temp (~20- 25C) in seconds? Acetic Acid Anhydride, 139. Ethylene Dichloride, 83. We assume a pressure of 1. The feed temperature will have to be adjusted to a value between the boiling points of acetone and isopropanol at a pressure of 1. Trade Tech by the various minority and majority.
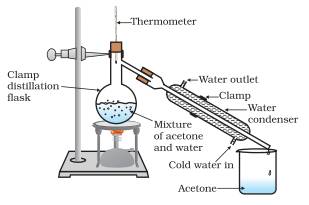
Water must be heated to a temperature of 212°F (100°C) in order for individual water molecules to get enough energy to break their hydrogen bonds to turn from a liquid to a gas. Chemists call this temperature the boiling point , the point at which a liquid becomes a gas. Take, ml of acetone and water mixture in 1:ratio, in a 1ml round bottomed flask. Fit the cork, thermometer and water condenser as shown in Fig.
Heat the acetone -water mixture slowly. Answer to The normal boiling point of acetone , an important laboratory and industrial solvent, is 56. The binary mixture of acetone ( boiling point ) and methanol ( boiling point ) is difficult to separate by distillation because of the presence of a minimum boiling azeotrope ( mole acetone at ). This Demonstration shows that MEK ( boiling point ) cannot be used as an entrainer to break this azeotrope at atm. Ultra-fine magnetic particles are difficult to be dispersed in low boiling point solvents such as alcohol (C1–C4) and acetone.
In this paper, we report the preparation methods of several alcohol and acetone -based magnetic fluids. The stability of magnetic fluid depended on the HLB (hydrophile–lipophile balance) of the .

Calculation Over Validity Range. Physical state and appearance: Liquid. Taste: Pungent, Sweetish. Critical Temperature: 235°C (455°F). Flash Point (Setaflash Closed Cup. -18°C (-°F). The density values are thought to be accurate .